II 2021
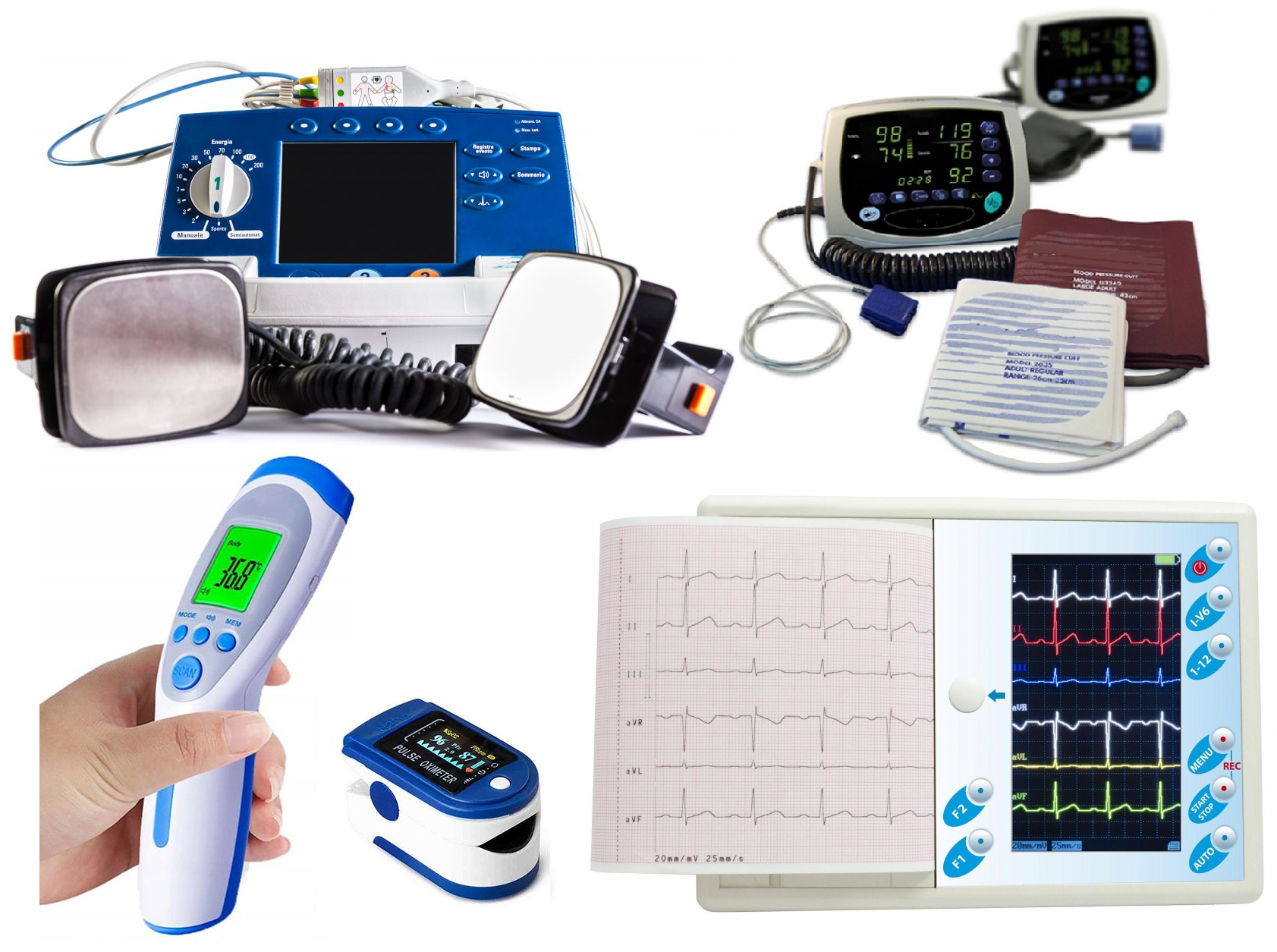
In our laboratory you will carry out safety and electromagnetic compatibility tests, among others the following medical equipment: EN 60601-2-10 Nerve and muscle stimulators EN 60601-2-18 Endoscopic devices EN 60601-2-22… Read More "II 2021
In our laboratory you will carry out safety and electromagnetic compatibility tests, among others the following medical equipment: EN 60601-2-10 Nerve and muscle stimulators EN 60601-2-18 Endoscopic devices EN 60601-2-22… Read More "II 2021
We have started the process of obtaining the status of a notified body in the scope of Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices. ... Read More "VIII 2020
In our laboratories we perform the following research and tests of medical devices in accordance with the ISO 10993 series standards: mutagenicity: bacterial reverse mutation test in vitro aberration tests ... Read More "IV 2020

ICR Poland on the basis of accreditation by PCA AC197 is the only Polish certification body competent to assess type A, B, C and D generating units, as required:
- Commission Regulation (EU) 2016/631 of 14 April 2016 establishing a Grid Code on requirements for the connection of generating units to the grid (OJ EU L 112/1 of 27.4.2016),
- General application requirements resulting from the EU Commission Regulation 2016/631 of 14 April 2016 laying down the Grid Code on requirements for the connection of generating units to the grid - approved by the Decision of the President of the Energy Regulatory Authority DRE.WOSE.7128.550.2.2018.ZJ of 2 January 2019.
Our certificates are issued on the basis of certification programme consistent with the document PTPiREE "Conditions and procedures for the use of certificates in the process of connecting generation modules to the electricity grid" - Version 1.2 of 28 April 2021 and Version 1.3 of 1 October 2024.
We encourage manufacturers and importers of power generation modules to make use of our services so that the products offered can be connected to power grids in Poland and the EU.
We invite you to contact.
ICR Poland, based on the AC197 accreditation granted by PCA, is the only Polish certification body with competence to assess type A, B, C and D generation units, according to the requirements of:
- COMMISSION REGULATION (EU) 2016/631 of 14 April 2016 establishing a network code on requirements for grid connection of generators (O.J. EU L 112/1 of 27.4.2016),
- General Application Requirements resulting from the Commission Regulation (EU) 2016/631 of 14 April 2016 establishing the network code on the requirements
for connecting generators - approved by the Decision of the President of the Energy Regulatory Office (URE) DRE.WOSE.7128.550.2.2018.ZJ of 2 January 2019.
Our Certificates are issued on the basis of a certification program in accordance with the PTPiREE document "Conditions and procedures for the use of certificates in the process of connecting energy generation modules to power grids" - Edition 1.2 of April 28, 2021 and Edition 1.3 of October 1, 2024.
We invite manufacturers and importers of power generation modules to use our services so that the products offered can be connected to power grids in the Poland and rest of EU.
Feel free to contact us.